
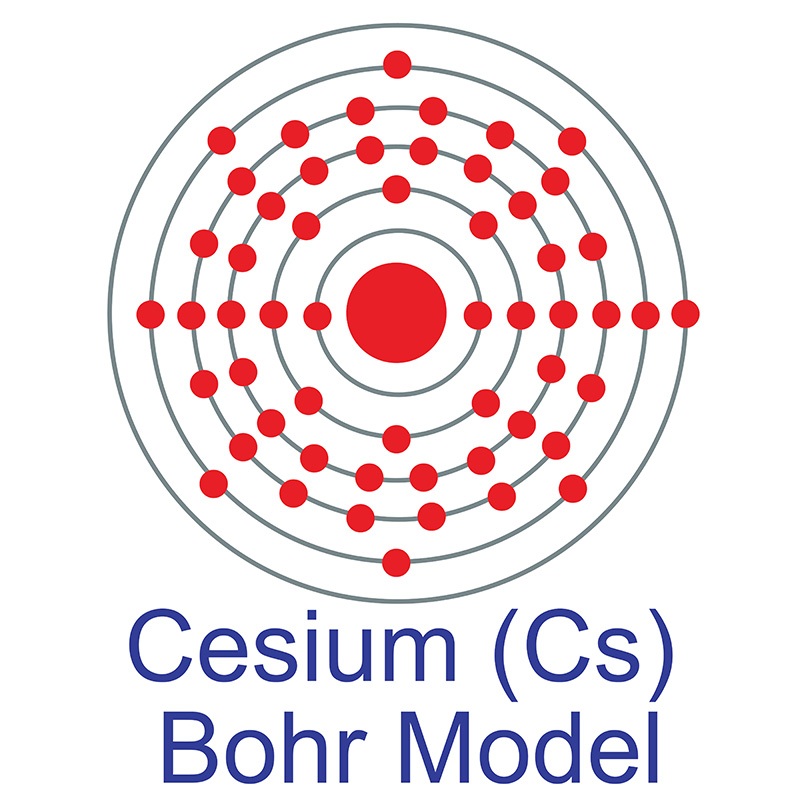
Cesium(Cs) electron configuration and orbital diagram
Cesium is silvery-gold, soft, ductile alkali metal. It is liquid in a warm room, melting at 28.4 o C (83.1 o F). Cesium is one of the few metals that is liquid near room temperature. The others are gallium, francium and mercury. Cesium is an extremely reactive metal and the most alkaline of the elements.

How To Find an Valence Cesium Electron Configuration (Cs)
The electron configuration of cesium consists of one lone 6s electron outside a perfectly symmetrical core of 54 other electrons characteristic of the noble element xenon. This fact has made it the foundation for mankind's best clock, the cesium atomic clock. Atomic data: Nuclear data: Index Periodic Table

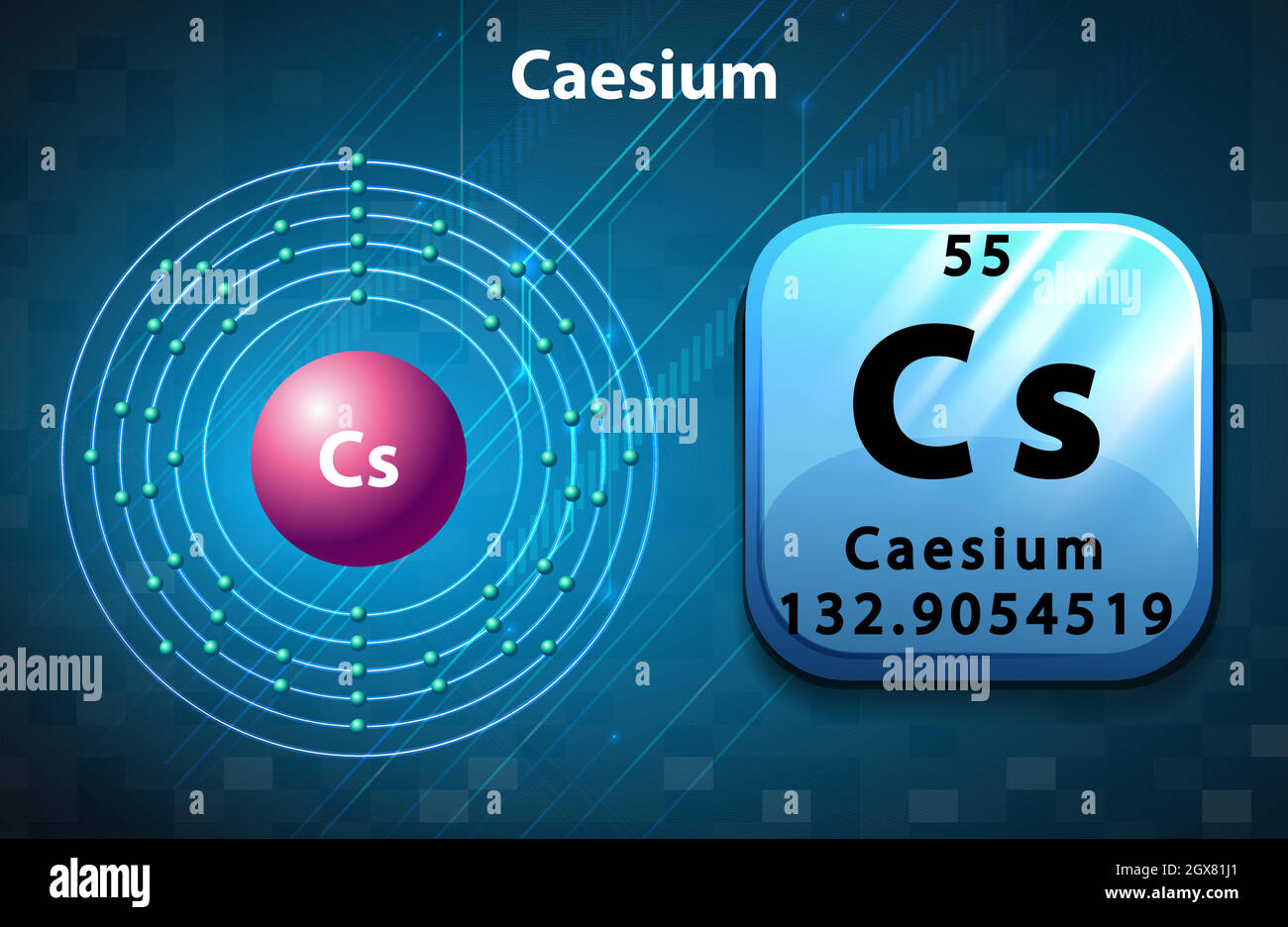
Caesium, atomic structure Stock Image C013/1605 Science Photo Library
Electron configuration for Cesium (element 55). Orbital diagram Cs (Cesium) is an element with position number in the periodic table. Located in the : 28.4 ℃. Electronic configuration of the Cesium atom in ascending order of orbital energies: Electronic configuration of the Cesium atom. Valence electrons. Orbital diagram

Caesium Atom Stock Illustration Download Image Now Atom, Balance
Caesium - Element information, properties and uses | Periodic Table Element Caesium (Cs), Group 1, Atomic Number 55, s-block, Mass 132.905. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
Cesium Atomic Orbital Diagram
Cesium Electronic configuration. Electronic configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 1 >> Back to key information about the elementBack to key information about the element
Cesium Cs (Elements 55) of Periodic Table Elements FlashCards
Full electron configuration of cesium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1 xenon ← cesium → barium Cesium, complete electron configuration.

Caesium, atomic structure Stock Image C023/2561 Science Photo Library
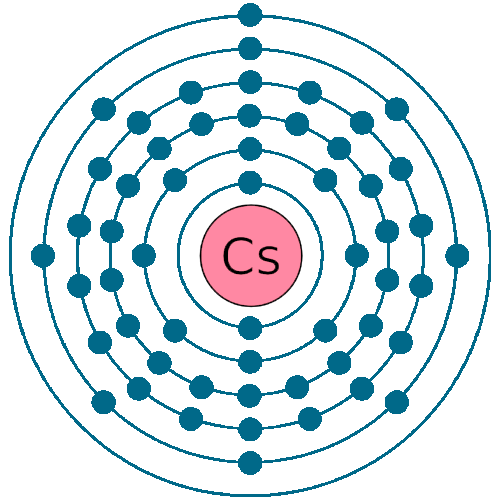
Caesium -. Cs: properties of free atoms. Caesium atoms have 55 electrons and the shell structure is 2.8.18.18.8.1. The ground state electron configuration of ground state gaseous neutral caesium is [ Xe ]. 6s1 and the term symbol is 2S1/2.

How to write the electron configuration for Cesium (Cs and Cs+) YouTube
0:00 / 2:59 How to write the electron configuration for Cesium (Cs and Cs+) Wayne Breslyn 723K subscribers Subscribe 29K views 3 years ago A step-by-step description of how to write the electron.
Symbol and electron diagram for Caesium Stock Vector Image & Art Alamy
Electron configurations can be predicted by the position of an atom on the periodic table.. For example, take the elements in the first column of the periodic table: H, Li, Na, K, Rb, and Cs. Their electron configurations (abbreviated for the larger atoms) are as follows, with the valence shell electron configuration highlighted: Electrons.

Symbol and electron diagram of Caesium Stock Vector Image & Art Alamy
Electrons and Electron Configuration The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Caesium is 55.
Cs Cesium Element Information Facts, Properties, Trends, Uses and
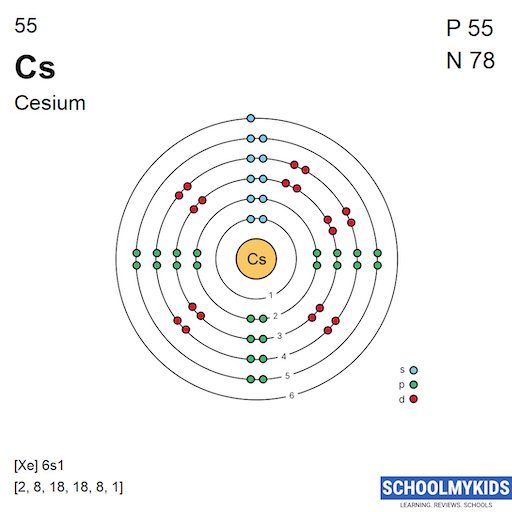
Caesium is a chemical element with atomic number 55 which means there are 55 protons and 55 electrons in the atomic structure. The chemical symbol for Caesium is Cs. Electron Configuration and Oxidation States of Caesium Electron configuration of Caesium is [Xe] 6s1. Possible oxidation states are +1. Electron Configuration
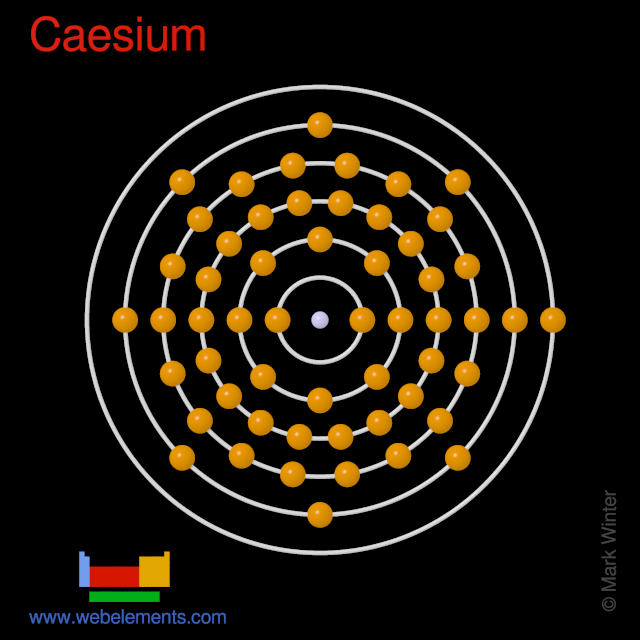
WebElements Periodic Table » Caesium » properties of free atoms
Electron Configuration For Cesium: Caesium ( Caesium is the IUPAC spelling) or cesium (Cesium is the American spelling) is a chemical element which has a chemical symbol Cs. The atomic number of cesium is 55. It is a silvery-gold soft, alkali metal that has a melting point of 83.3 °F ( 28.5 °C), that makes it one of only five elemental metals which are liquid near or at […]

WebElements Periodic Table » Caesium » properties of free atoms
Electron configuration 6s 1: Electrons per shell: 2, 8, 18, 18, 8, 1. Caesium (IUPAC spelling; cesium in American English) is a chemical element; it has symbol Cs and atomic number 55.. Caesium is also important for its photoemissive properties, converting light to electron flow.
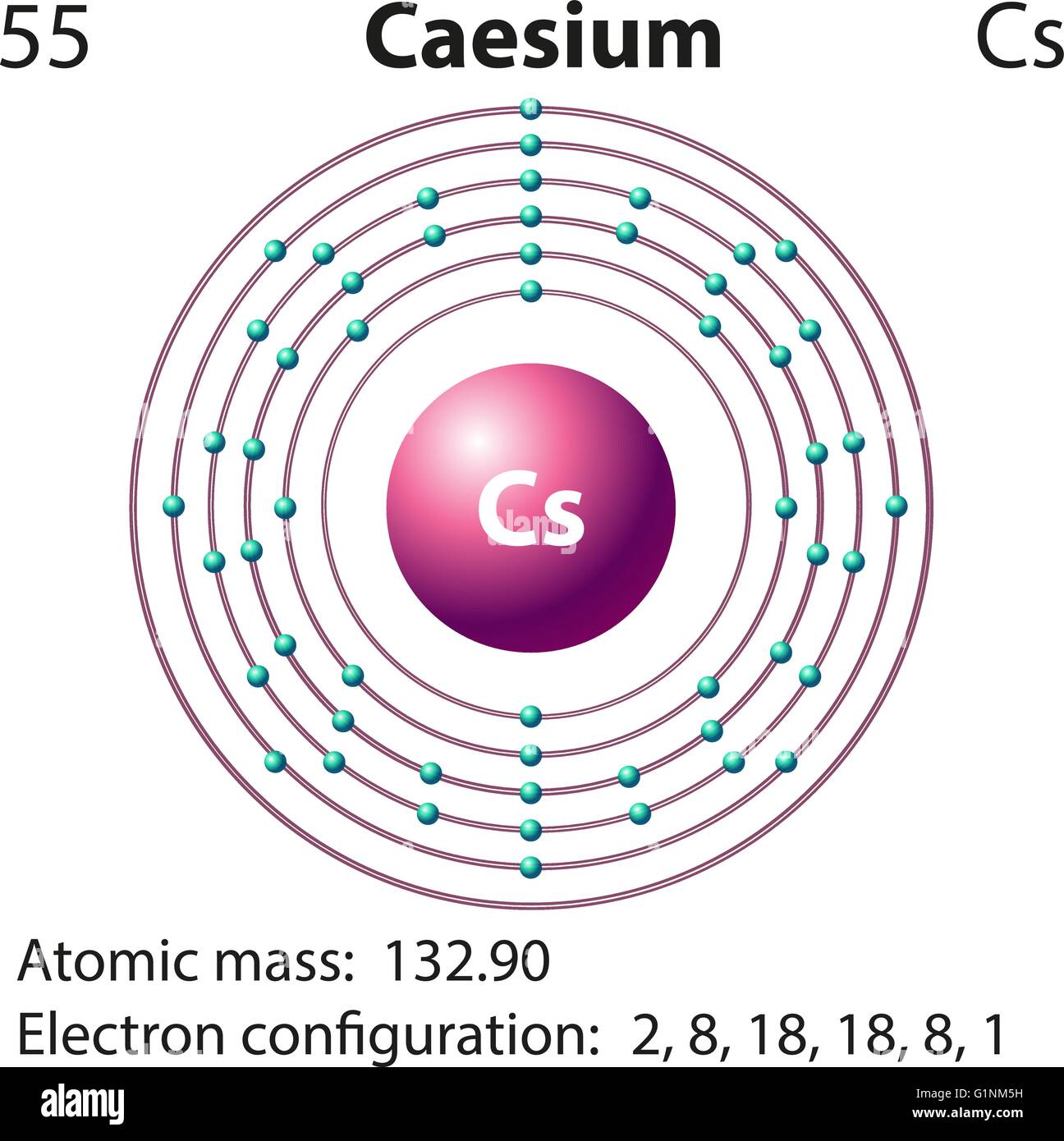
Symbol and electron diagram for Caesium illustration Stock Vector Image
The Electron: Crash Course Chemistry #5. Video 3.1.2 3.1. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).

Cesium(Cs) electron configuration and orbital diagram
Electron configuration: [Xe]6s1 Oxidation state: 1 Crystal structure: cubic

Cesium Electron configuration Symbol Atomic Number Atomic Mass
Caesium Electron Configuration Caesium has the occurrence form from the mining of pollucite and also has some other minor forms of occurrence. The chemical element was first discovered by German scientists in the year of 1860. It's quite an old chemical variant in itself that is useful for a number of practical domains.