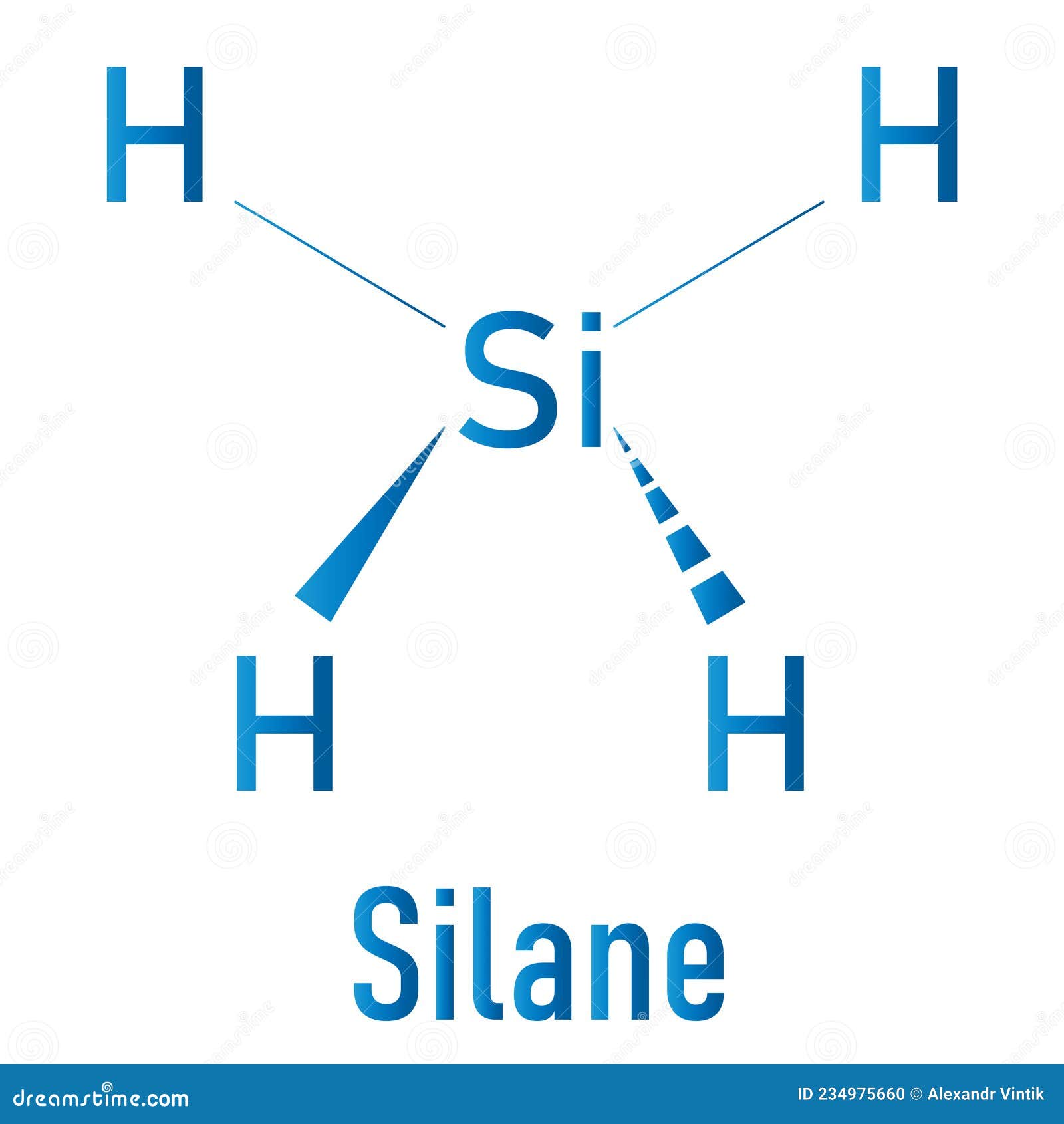
Silane SiH4 Molecule. Skeletal Formula Stock Vector Illustration of
SiH4 Lewis Structure Lewis Structure is a two-dimensional diagrammatic approach towards finding the nature of chemical bonding present inside any given molecule. Here, we use dot notations to represent the electrons, and hence this is also known as the electron-dot structure.

SiH4 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
So that is the SiH4 Lewis structure. This is Dr. B., and thanks for watching. Search our 100 + Lewis Structures (Opens New Window) See the Big List of Lewis Structures : Frequently Tested Lewis Structures Basic CH 4, NH 3, C 2 H 4, O 2, N 2 Intermediate O 3, BBr 3, I 3-, BrF 5, NO

como se forma el sih4 a partir de sus atomos utilizando la estructura
Hello everyone! Welcome back to our channel, and in today's video, we will help you do SiH4 Lewis Structure. Follow this video to know the detailed method an.
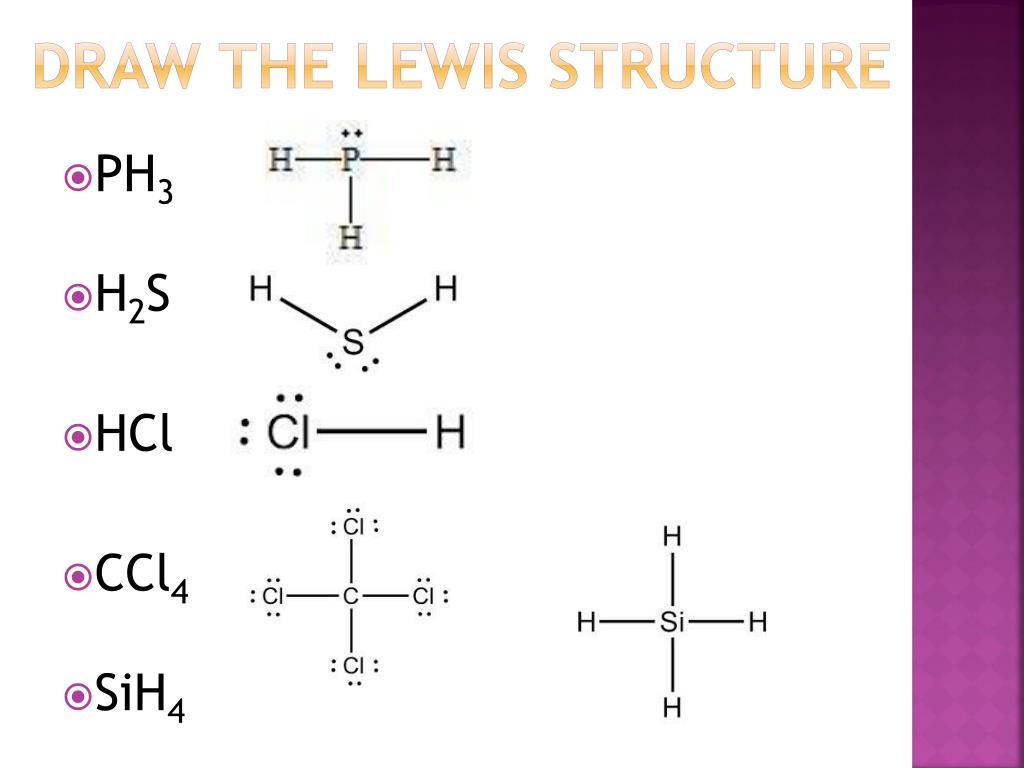
PPT Covalent Bonds PowerPoint Presentation, free download ID3048466
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for silane (SiH4).

SiH4 Lewis Structure (Silicon Tetrahydride) YouTube
By Darshana Fendarkar In this article,"SiH4 Lewis structure", different facts like Lewis structure drawing, formal charge calculation, hybridization, structure with some detailed explanations are described below. SiH4 is also called silane or monosilane, it is a colorless flammable and poisonous gas with a strong pungent odor.

SiH4 Lewis Structure (in 6 Steps With Diagrams) Study Striver
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrafluoride).For the SiH4 structure use the periodic table to find the tota.
8+ ford escape air conditioning diagram SenoTybalt
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons:. {SiH4}\): Si already has an octet, so nothing needs to be done.

Solved SiH4 1) Silane Lewis structure Electron pair geometry
SiH4 Lewis Structure - How to Draw the Lewis Structure for SiH4 (Silicon Tetrahydride) Watch on So from the above diagram we have come to know that the SiH4 molecule has four Si-H bonds. Now in the next step we have to check whether these four Si-H bonds are polar or nonpolar. And we also have to check the molecular geometry of SiH4.
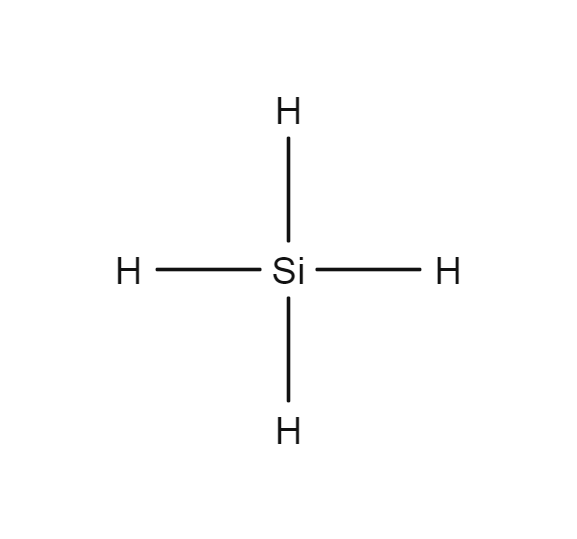
Silane Lewis Structure
An explanation of the molecular geometry for the SiH4 (Silicon Tetrahydride (Silane) including a description of the SiH4 bond angles. The electron geometry f.

Is SiH4 Polar or Nonpolar? (Silicon Tetrahydride) YouTube
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 structure use the periodic table to find the total.
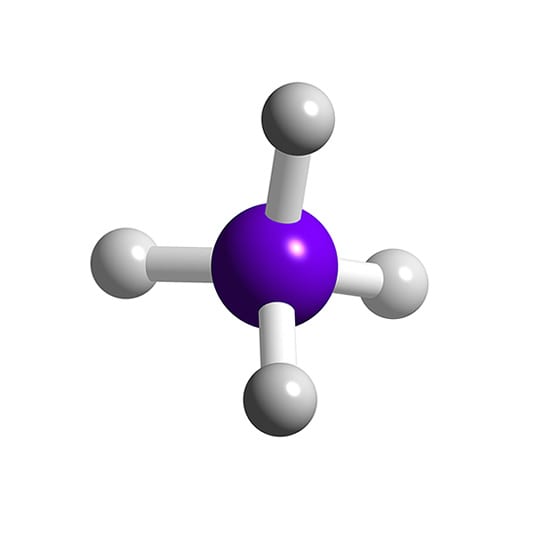
SiH4 Silane
The Lewis structure indicates that each [latex]\ce{Cl}[/latex]. {CCl4}[/latex] (carbon tetrachloride) and silicon in [latex]\ce{SiH4}[/latex] (silane). Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule. The transition elements and inner transition elements also do not follow the octet rule:

Silicon(IV) iodide, 99.999 (metals basis), Thermo Scientific Chemicals
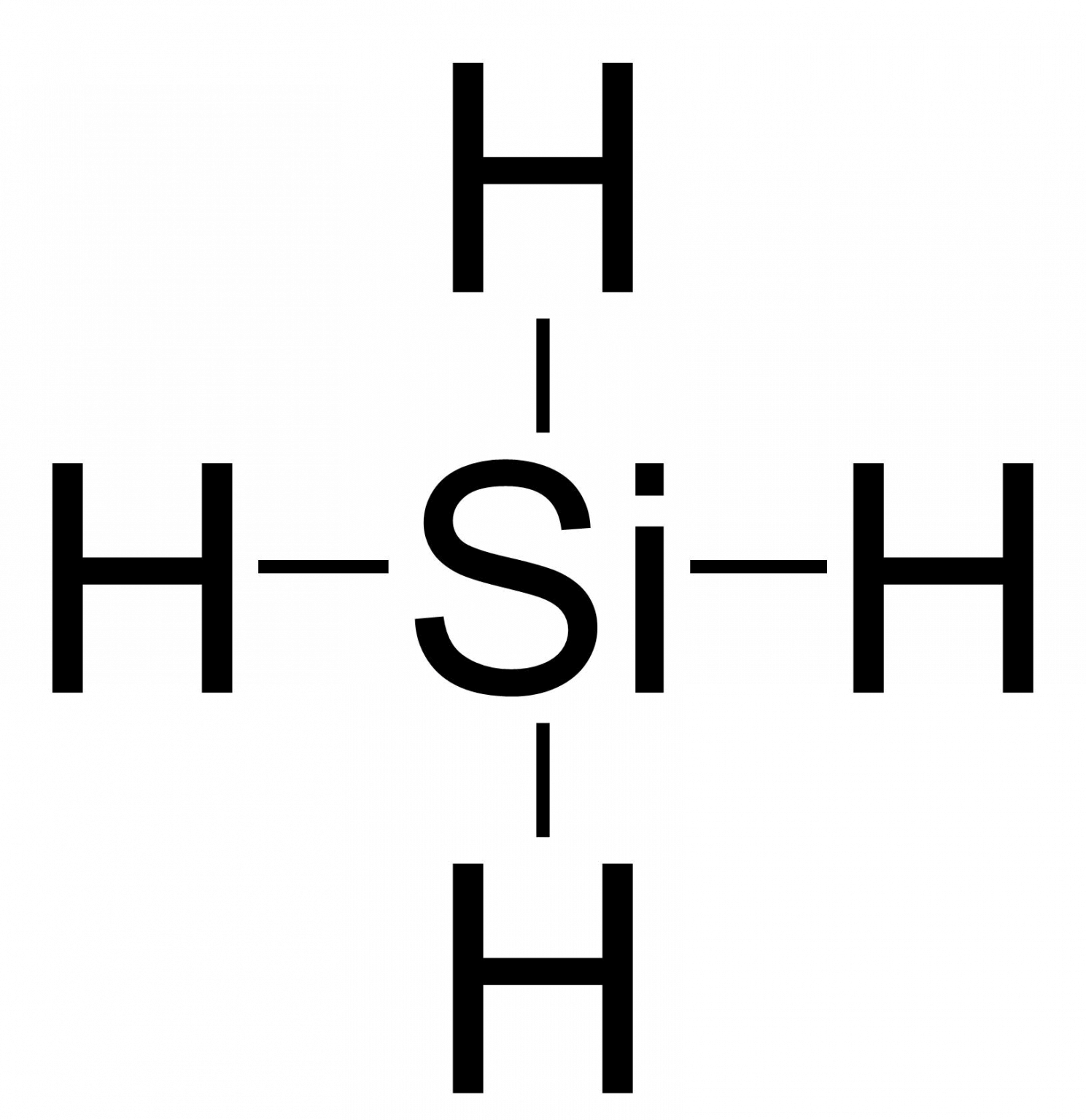
SiH4 lewis structure has a Silicon atom (Si) at the center which is surrounded by four Hydrogen atoms (H). There are 4 single bonds between the Silicon atom (Si) and each Hydrogen atom (H).

Silane (SiH4) molecule. Skeletal formula Stock Vector Image & Art Alamy
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures

El Diagrama De Lewis
Dipole Moment 9m. Octet Rule 6m. Formal Charge 2m. Lewis Dot Structures: Neutral Compounds 8m. Lewis Dot Structures: Sigma & Pi Bonds 4m. Lewis Dot Structures: Ions 9m. Lewis Dot Structures: Exceptions 6m. Lewis Dot Structures: Acids 3m. Resonance Structures 12m.
[Solved] Draw the Lewis structure for SiH 4 in the window below and
Draw the Lewis structure for SiH4. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. Include all hydrogen atoms. To change the symbol of an atom, double-click on the atom and enter the letter of the new atom. PART B Draw the Lewis structure for CO.
M8Q2 Lewis Structures Chem 103/104 Resource Book
Structure Chemical Safety Laboratory Chemical Safety Summary (LCSS) Datasheet Molecular Formula H4Si SiH4 Synonyms 5J076063R1 7803-62-5 silane Silicane SiH4 View More. Molecular Weight 32.117 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2004-09-16 Modify: 2023-12-30 Description