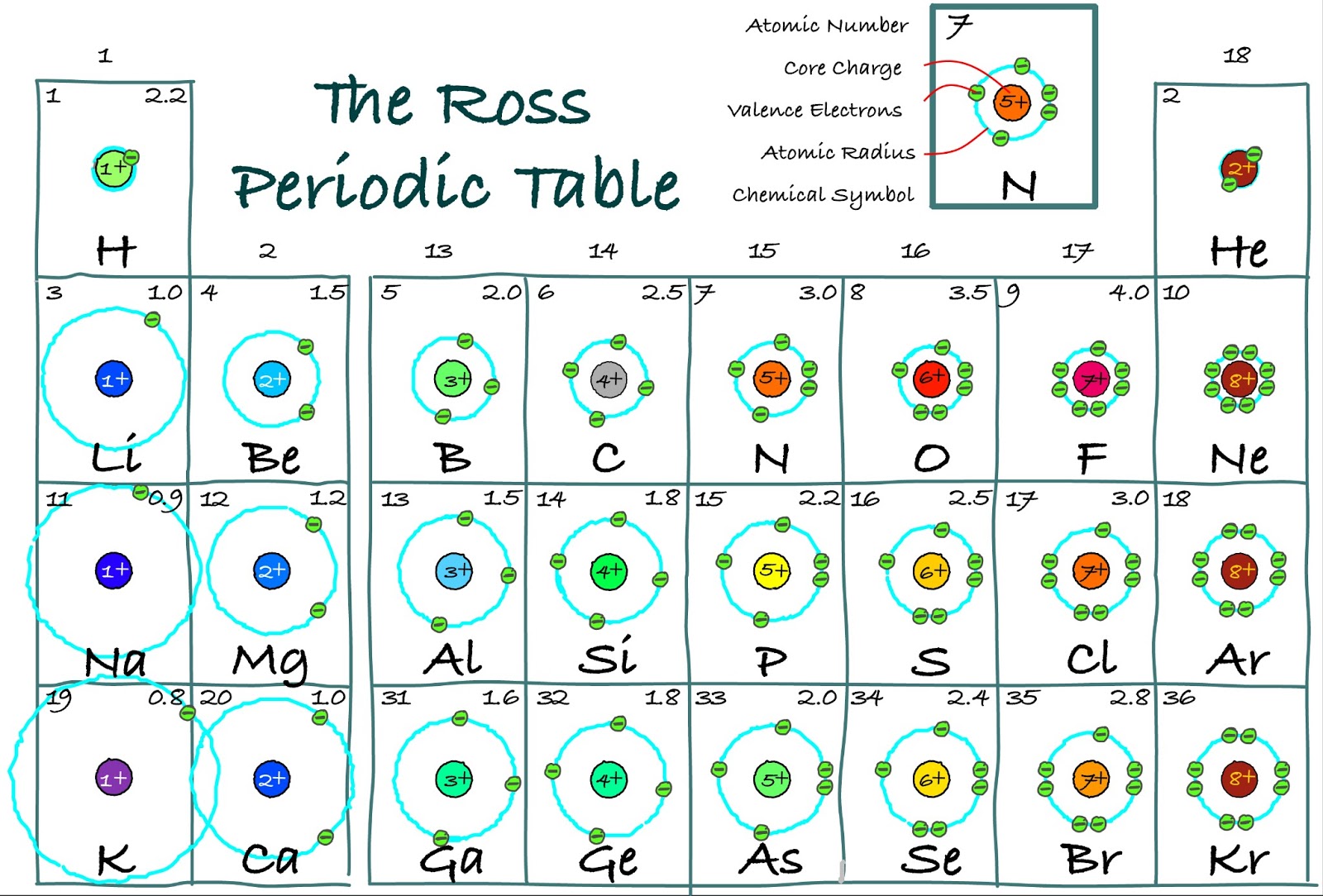
Electron arrangements
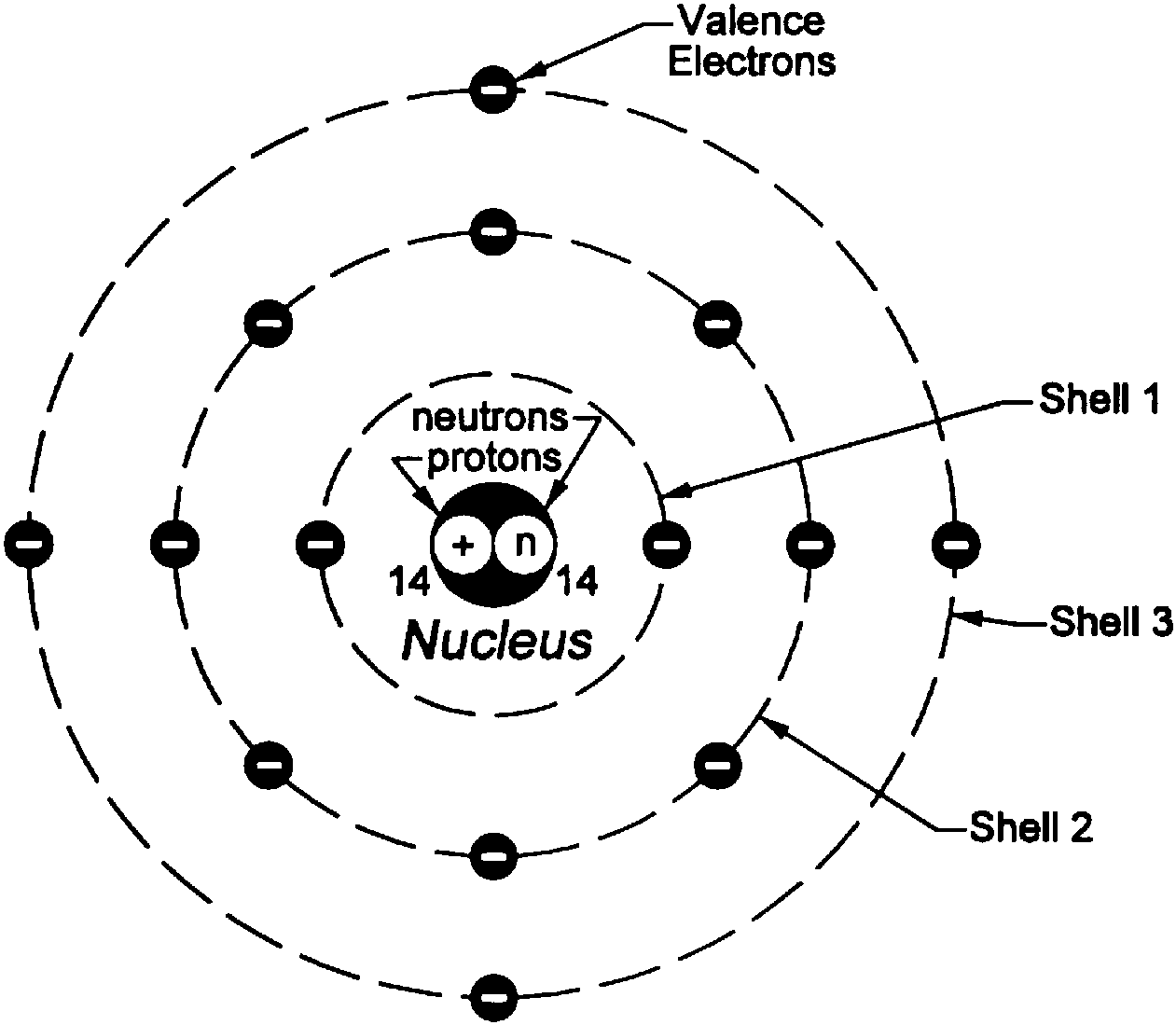
Bohr diagram or Bohr rutherford diagram describes the visual representation of orbiting electrons around the small nucleus. It used different electron shells such as K, L, M, N…so on.
Bohr Model Silicon Atom Electron Structure Stock Vector (Royalty Free
Manish Bhardwaj. 5.6: Bohr Diagrams of Atoms and Ions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells,..

Number of valence electrons in silicon
The drawbacks of the Rutherford model of the atom, including its apparent instability, would soon be addressed by his student, Dutch physicist Neils Bohr (1885 - 1962). To unlock this lesson you.
Symbol and electron diagram for silicon Royalty Free Vector
draw a Bohr-Rutherford diagram for sulfur. draw a Bohr-Rutherford diagram for chlorine. draw a Bohr-Rutherford diagram for argon. draw a Bohr-Rutherford diagram for potassium. draw a Bohr-Rutherford diagram for calcium. Identification of elements from Bohr-Rutherford Diagram. Learn with flashcards, games, and more — for free.
Silicon Bohr Model Diagram, Steps To Draw Techiescientist
How to Draw the Bohr-Rutherford Diagram of Silicon chemistNATE 258K subscribers Subscribe Subscribed 245 22K views 4 years ago Silicon has 2 electrons in its first shell, 8 in its second, 4 in.
_0.jpg?itok=RuBFwyf1)
Le modèle atomique de RutherfordBohr Alloprof
Bohr Diagram: The First Element In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen 1 proton 1 electron 0 neutrons
valence electrons of silicon
Mr. Primmer Demonstrates How to Draw Bohr Rutherford Diagrams!

Image result for silicon atomic model
4) The following diagram is a Bohr-Rutherford diagram of one element from the periodic table: To which group and period does this element belong? A) Period 3 group 4. B) Period 4 group 4. C) Period 3 group 1. D) Period 1 group 3. 5) Referring to the periodic table, find an element that has the same number of electron shells and
FileThis shows the bond of Silicon Oxide using the Bohr model.png
The Bohr Model of Silicon (Si) has a nucleus that contains 14 neutrons and 14 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Silicon contains 4 electrons also called valence electrons. Page Contents show How to draw Bohr Model of Silicon (Si)?

View 19 Silicon Element Bohr Model aboutdiecolor
In this video we'll look at the atomic structure and Bohr model for the Silicon atom (Si). We'll use a Bohr diagram to visually represent where the electrons.
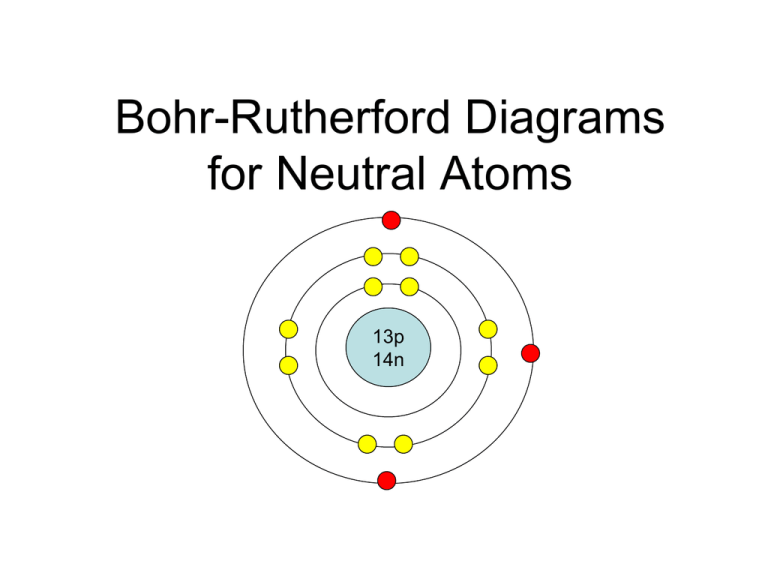
BohrRutherford diagrams for atoms
Draw a molecule of SiS 2, using Bohr-Rutherford diagrams showing all electrons (core electrons and valence electrons). Ensure that all atoms in the molecule have a full valence shell. Show your work. Draw a molecule of SiS 2 as a Lewis dot diagram, showing all valence electrons; Answer the following questions about the molecule you drew in.
Bohr model of silicon atom Electronics And Engineering Lab
Contents show Bohr Model of Silicon The Bohr model is also known as the Bohr-Rutherford model as it was developed as a modification of the Rutherford model. It was given by Niel Bohr in 1913. This model is used to illustrate the atomic composition in pictorial form.
Bohr Model For Silicon
This page contains materials for the session on the atomic models of Rutherford and Bohr. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.
What Is A Bohr Diagram Hanenhuusholli
The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915.
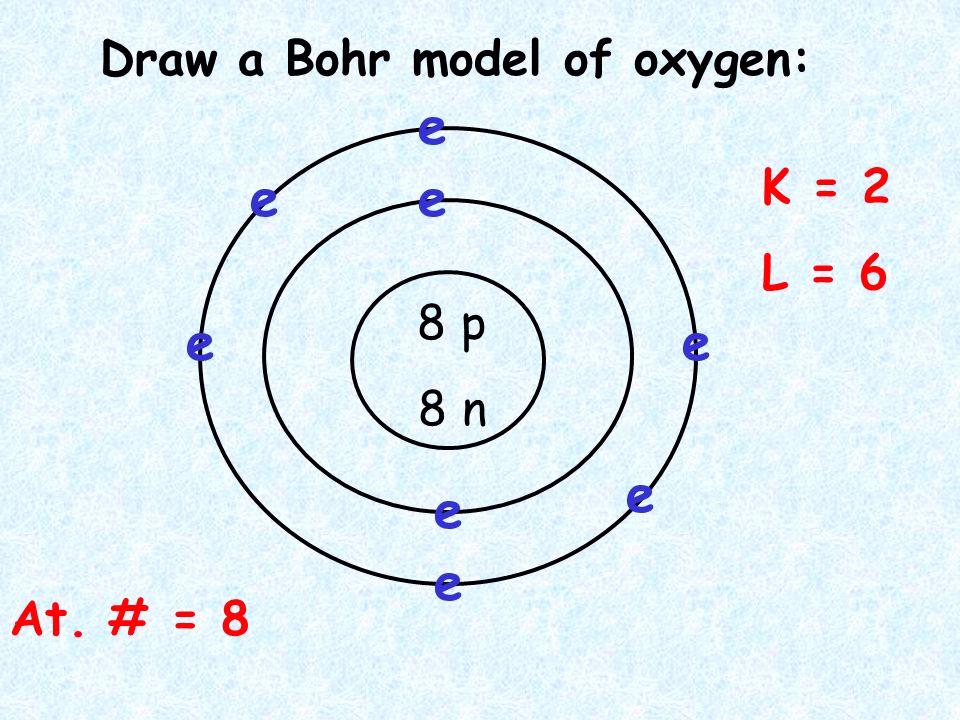
Diagram Bohr Model Periodic Table Periodic Table Timeline
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Silicon Si (Element 14) of Periodic Table Elements FlashCards
2. What elements is represented by this diagram? How do you know? 3. What is the charge of this nucleus? What is the charge of this atom overall? 4. What is the mass of this atom? 5. Using a periodic table, look up titanium. a. What is its atomic number? b. How many protons does a titanium atom have? c. How many electrons does it have? 6. What.