
What is Oxygen? Definition, Properties, Elements, Uses
The first thing we can do is check all of our diagrams depict eight protons and eight electrons. All the nuclei look identical, and they each have eight protons. This means we're dealing with nuclei of oxygen, and we can proceed to the next test. The easiest way to count out electrons is to work out the electron configuration of each diagram.

Oxygen, atomic structure Stock Image C018/3689 Science Photo Library
2. Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides. [3]
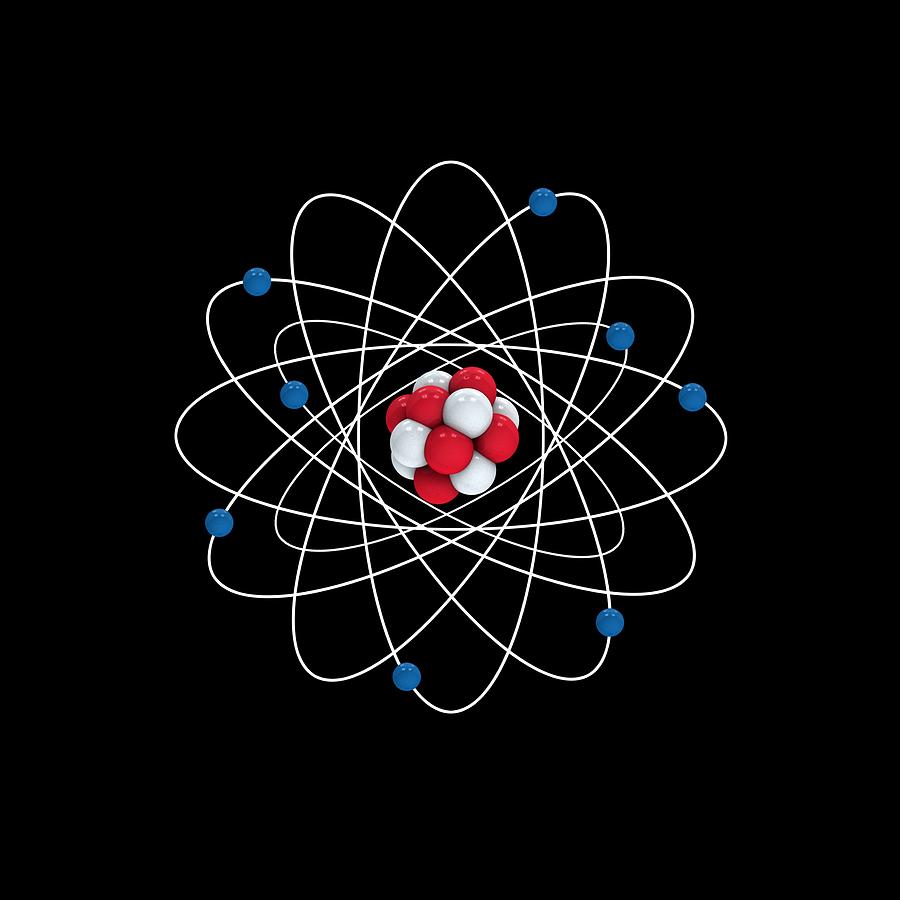
Oxygen atomic structure, artwork Photograph by Science Photo Library Pixels
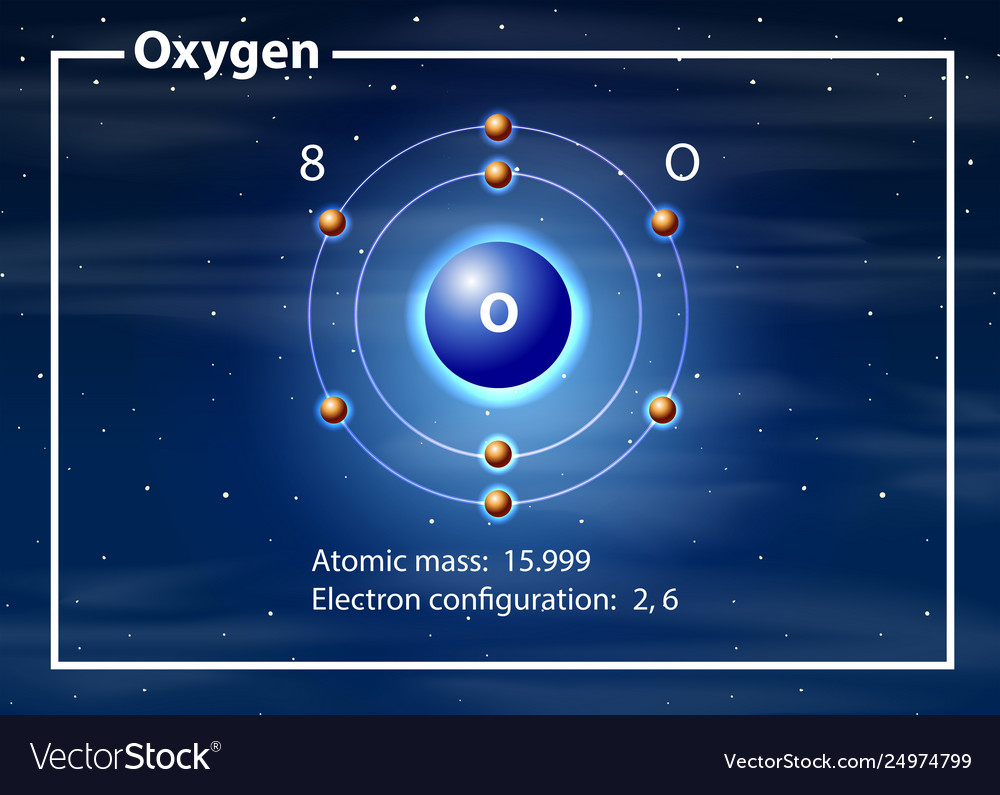
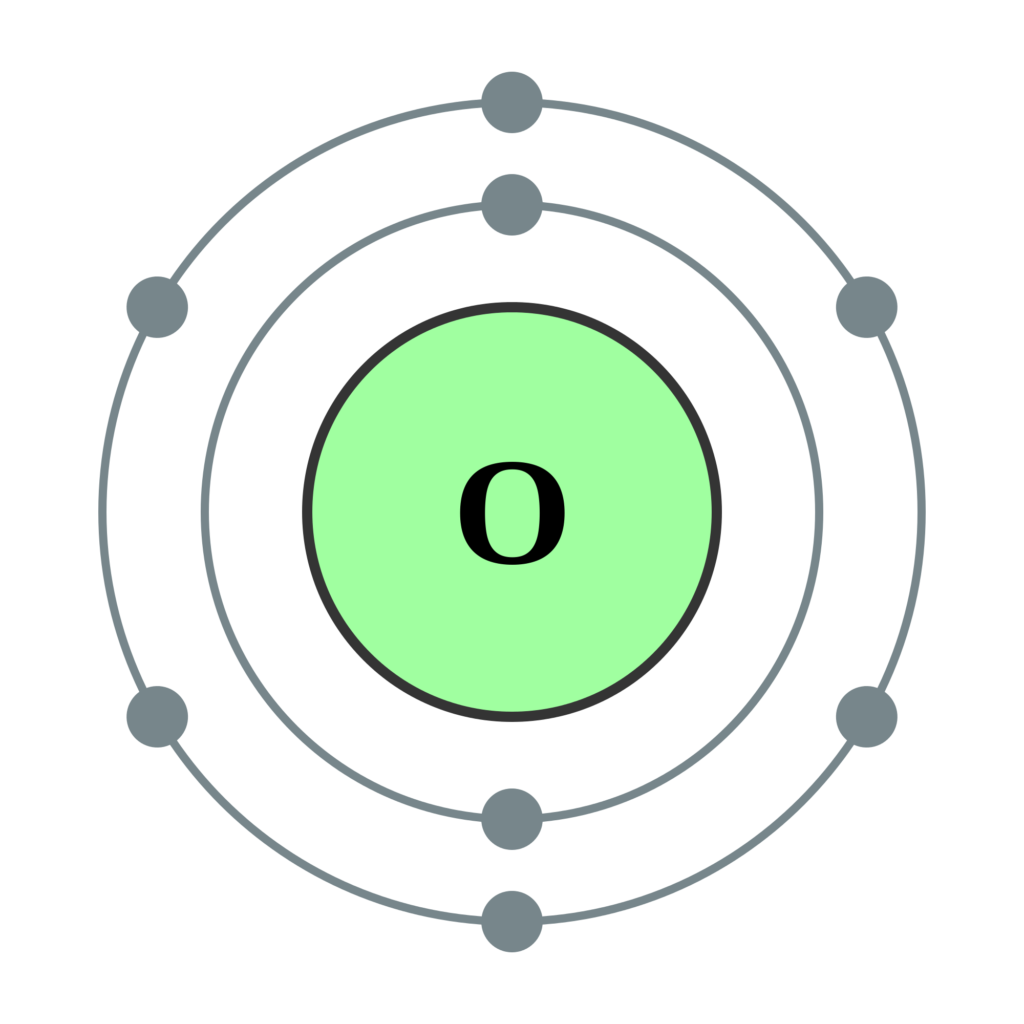
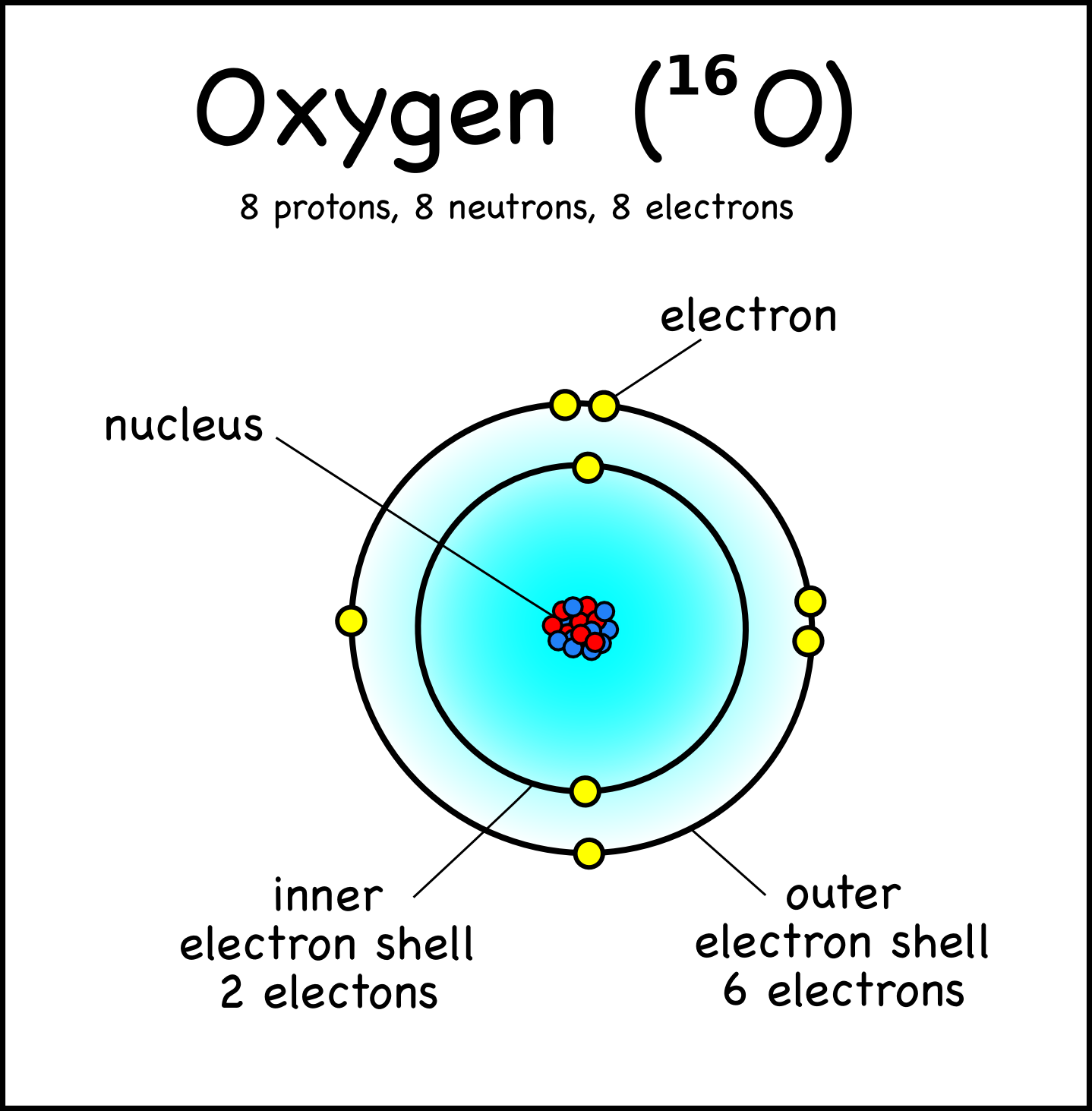
8 neutrons To know more about the atomic structure of oxygen, you need to learn about the electronic configuration. The electronic configuration shows the distribution of electrons in an atom. And, it can be shown in two ways: In the form of shells In the form of orbitals Let's talk about orbitals first.

Oxygen Atom Science Notes and Projects
CAS Registry Number: 7782-44-7. Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file. The 3d structure may be viewed using Java or Javascript . Other names: Molecular oxygen; Oxygen molecule; Pure oxygen; O2; Liquid oxygen; UN 1072; UN 1073; Dioxygen.

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
Magnetic Properties of Oxygen. Oxygen (O 2) is paramagnetic.An oxygen molecule has six valence electrons, so the O 2 molecule has 12 valence electrons with the electron configuration shown below:. As shown, there are two unpaired electrons, which causes O 2 to be paramagnetic. There are also eight valence electrons in the bonding orbitals and four in antibonding orbitals, which makes the bond.
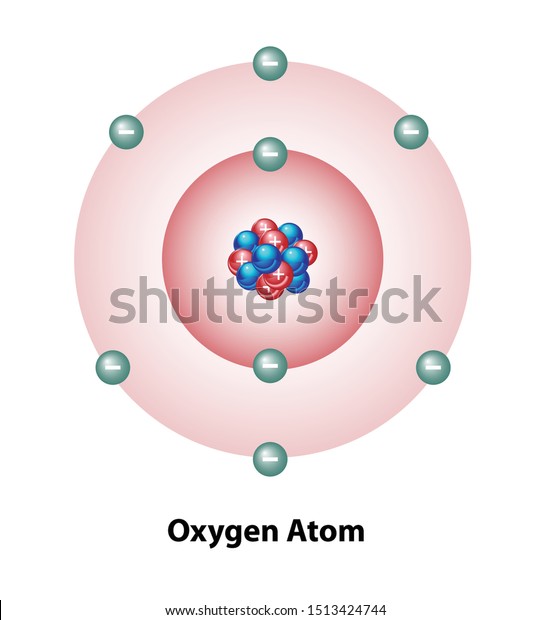
Diagram Oxygen Atom Nucleus Shells Protons Stock Vector (Royalty Free) 1513424744
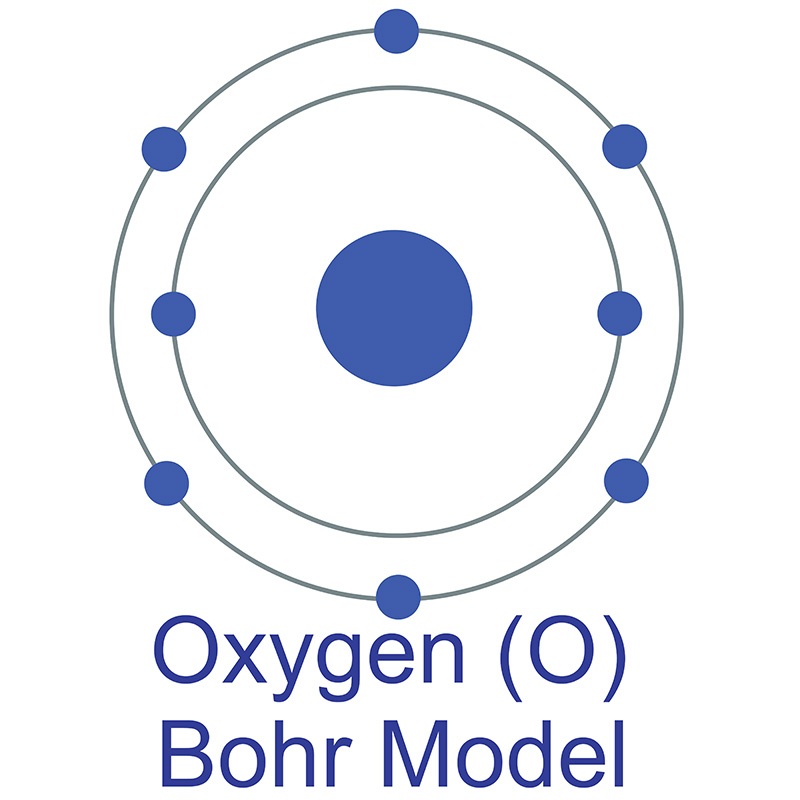
Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Oxygen atom with some simple steps. Steps to draw the Bohr Model of Oxygen atom 1. Find the number of protons, electrons, and neutrons in the Oxygen atom
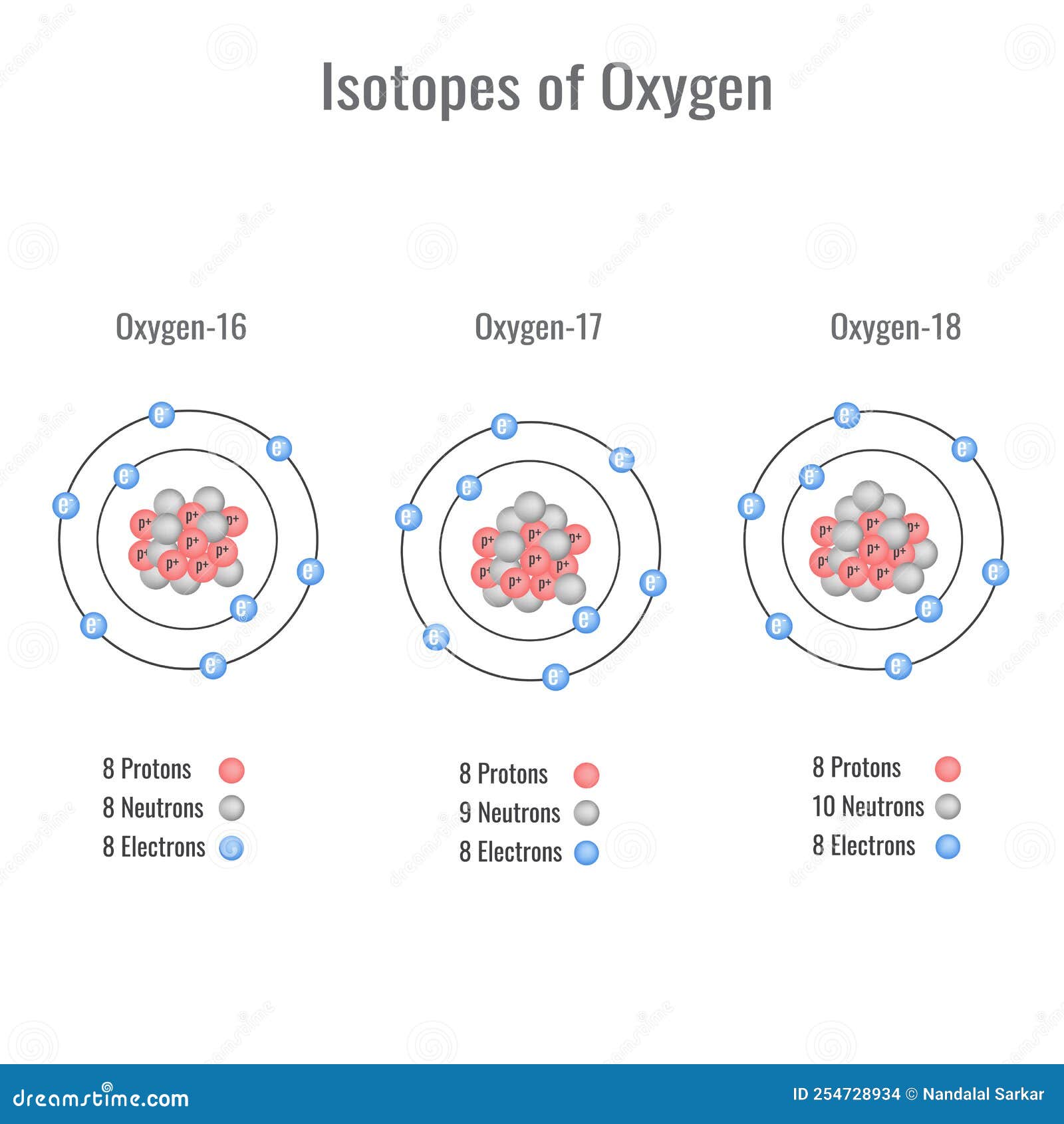
Isotopes of Oxygen Vector Illustration Stock Vector Illustration of atomic, education 254728934
oxygen (O), nonmetallic chemical element of Group 16 (VIa, or the oxygen group) of the periodic table. Oxygen is a colourless, odourless, tasteless gas essential to living organisms, being taken up by animals, which convert it to carbon dioxide; plants, in turn, utilize carbon dioxide as a source of carbon and return the oxygen to the atmosphere.
Forms of Energy ND Studies Energy Level 2
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. There are also two more valence electrons and they are paired.
Oxygen atom diagram concept Royalty Free Vector Image
In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate.
What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
The structure in which the hexagon portions connect through hexagonal prisms is faujasite, NaCa 0.5 (Al 2 Si 5 O 14)]. Since a terminal oxygen atom is bonded to each phosphorus atom, the coordination polyhedron of oxygen is also a tetrahedron. When the molecular P 4 O 10 is heated, a vitrified isomer is formed. This is a polymer composed of.

oxygen atom Chuba Oyolu's Portfolio
In this video we'll look at the atomic structure and Bohr model for the Oxygen atom (O). We'll use a Bohr diagram to visually represent where the electrons a.
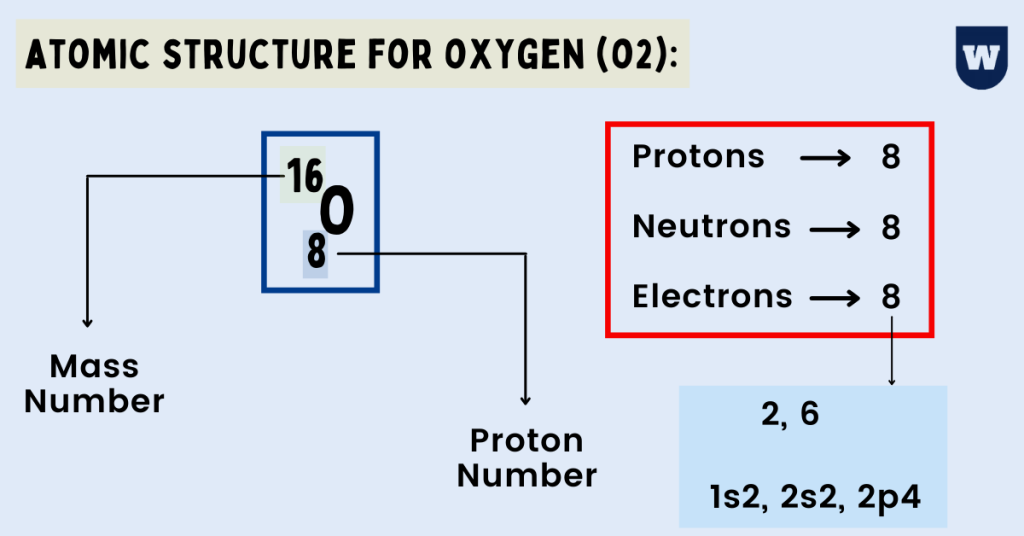
Atomic Structure for Oxygen (O2) Best Guide (With Diagrams)
¡Solo hoy, disfruta de todas las categorías hasta un 90% de descuento en tu compra. ¡Precios increíbles y alta calidad aquí en Temu. Envío gratuito en todos los pedidos
Drawing Atoms Montessori Muddle
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be.
Oxygen (O) AMERICAN ELEMENTS
Find the deal you deserve on eBay. Discover discounts from sellers across the globe. We've got your back with eBay money-back guarantee. Enjoy Oxygen' you can trust.

Electron Configuration Of Oxygen In Ground State
To write the orbital diagram for the Oxygen atom (O) first we need to write the electron configuration for just O. To do that we need to find the number of.

Diagram representation of the element oxygen Vector Image
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.